Abstract
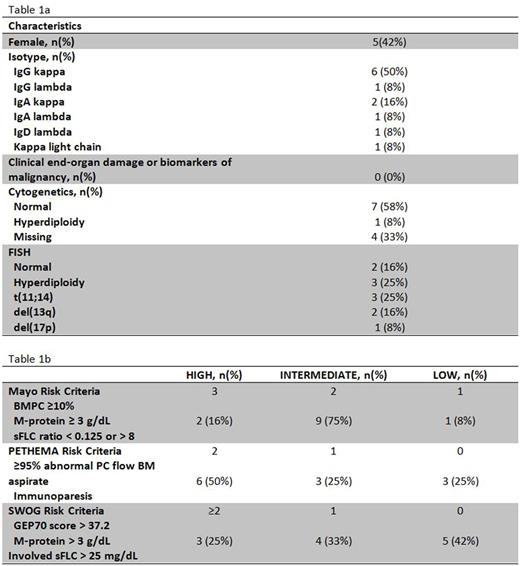
Background: The current management of patients with smoldering multiple myeloma (SMM) is watchful waiting. However, a fraction of these patients are at significant risk of progression to symptomatic multiple myeloma (MM). In a recent study, expression of PD-L1 on tumor cells correlated with increased risk of progression from SMM to MM (Dhodapkar et al Blood. 2015; 126(22): 2475-2478) suggesting a role for immune checkpoint blockade for immunoprevention of MM. Pembrolizumab is a humanized monoclonal antibody against programmed death receptor-1 (PD-1) and blocks the interaction between PD-1 and PD-L1. Here, we report the results of a pilot study evaluating the safety and efficacy of pembrolizumab in intermediate- and high-risk SMM patients (I-HR-SMM). Methods: Patients with I-HR-SMM by PETHEMA, Mayo or SWOG criteria were eligible. Permbrolizumab was administered at a dose of 200 mg IV every 21 days for up to 8 cycles. Patients were eligible to continue treatment for up to 24 cycles if they achieved ≥ minor response after 8 cycles. The primary objective was the overall response rate (ORR) after 8 cycles. The target ORR was 25%. The trial was conducted by the Simon's Minimax design and the ORR was estimated accordingly (Simon, 1989). Twelve patients were planned for the first stage. If at least one patient had ≥ partial response, 4 more patients could enroll for a total of 16 patients. Serial peripheral blood mononuclear cells and bone marrow samples were collected for comprehensive immunophenotyping using multiparametric flow cytometry, and molecular profiling with gene expression profiling (GEP) and exome sequencing. Results: Twelve patients with I-HR-SMM were dosed on the first stage between 08/2016 and 06/2017 (Table 1a/b). Median age was 67.5 years (range 49-74). Median number of cycles received was 8 (range 3-8) and all 12 were evaluable for safety and efficacy. Median follow up was 8.54 months (4.7 to 9.6 months). One patient achieved a stringent complete remission (sCR) (8%), ten patients had stable disease (83%), and one patient had progressive disease (8%). The patient with sCR had HR-SMM by GEP70 and FISH (deletion 17p and amplification of CKS1B) and 50% plasma cells infiltration in the bone marrow at study entry. The sCR with negative flow cytometry on bone marrow was noted in this patient after only 3 cycles of therapy and response is ongoing 8 months after the end of treatment. Five patients discontinued therapy due to related adverse events: grade 2 elevation in LFT (n=2), grade 3 myalgia (n=1), and grade 3 acute kidney injury (AKI) due to tubulo-interstitial nephritis (n=2) [all resolved with discontinuation of treatment or prednisone administration]. There were no grade 4/5 adverse events. Enrollment on second stage is ongoing. Imunophenotyping of paired baseline and end of treatment bone marrow samples showed that the patient who achieved sCR had high levels of PD-1 expression on CD4+ and CD8+ T cells at baseline and showed evidence of CD8+ T-cell activation after therapy with greater upregulation of CD69, CTLA-4, PD-1, TIM-3, 4-1BB, and ICOS relative to non-responders. In contrast, expression of other immune checkpoint molecules such as TIM-3, LAG-3, and CD244 were greater on T cells in non-responders at baseline relative to the responder suggesting greater exhaustion of T cells in non-responders. Molecular profiling including GEP, exome sequencing, and neoantigens analysis is ongoing and will be presented at the meeting. Conclusions: This pilot study of pembrolizumab monotherapy showed that it is tolerable and can induce sCR in patients with SMM. More importantly, it suggested that immunoprevention to prevent progression of SMM to MM is possible. Additional biomarker studies are needed to identify patients that are most likely to respond to this approach. Our correlative studies also provide insights into potential future combination approaches to further improve efficacy of immunoprevention strategies in SMM.
Manasanch: takeda: Consultancy; celgene: Consultancy; sanofi: Research Funding; quest diagnostics: Research Funding; merck: Research Funding; adaptive biotechnologies: Consultancy. Lee: Eutropics Pharmaceuticals: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Pimera Inc: Consultancy; Celgene: Consultancy; Daiichi Sankyo: Research Funding; Takeda: Consultancy. Patel: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy; Celgene: Consultancy. Thomas: Celgene: Research Funding; Bristol Myers Squibb: Research Funding. Neelapu: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karus: Research Funding; Cellectis Inc.: Research Funding; Poseida Therapeutics, Inc: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding. Orlowski: BioTheryX: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.